Atoms form chemical bonds in order to obtain stability. One type of chemical bond is ionic bonds. Ionic bonds are attractions between cation and anions. A substance with ionic bonds is an ionic compound. Ionic compounds are also known as salts.
Properties of Ionic Compounds (Salts):
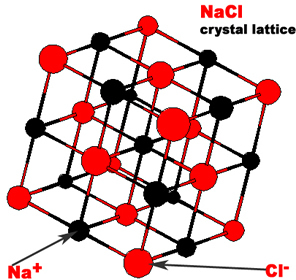
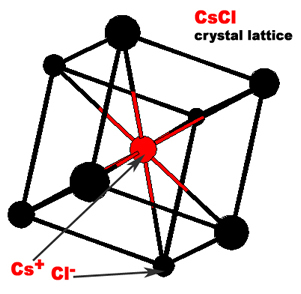
Ionic compounds are composed of a cation (positively charged atom) and an anion (negatively charged atom) in an orderly arrangement. The three dimensional arrangement of atoms or ions in a crystal is referred to as crystal lattice. The crystal lattices of sodium chloride and cesium chloride are shown below.
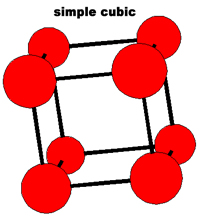
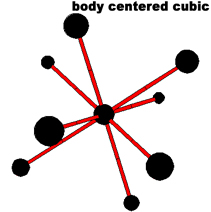
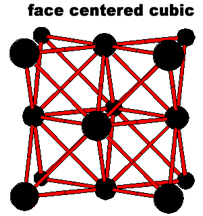
The simplest repeating unit of a crystal is known as a unit cell. Both NaCl and CsCl are classified as part of a cubic crystal system. The unit cells of NaCl and CsCl are different because their ions have different sizes. Three different ways that atoms can arrange themselves in a cubic crystal system are shown below.
Nearly all ionic compounds are crystalline solids at room temperature.
Most ionic compounds dissolve in water.
Ionic compounds conduct electricity when molten or dissolved in water. Solid ionic compounds do not conduct electricity because in order for a substance to conduct electricity it must have charged particles that can move freely. Ions in ionic compounds cannot move very much, except to vibrate.
Ionic compounds do not have an overall net charge. They are electrically neutral because the amount of positive charge is equal to the amount of negative charge.
Ionic compounds have higher melting and boiling points compared to other types of compounds (covalent compounds) because the ions in an ionic compound form strong bonds with a number of different ions due to their arrangement into crystalline structures.
Ionic compounds are hard and brittle because their ions are arranged into unit cells which form layers. As long as the layers stay aligned, the ionic compound is hard. But, if one layer is shifted, like charges will be next to one another. The repulsive forces between like ions causes the layers to break apart. |